1 Which of the Following Compounds Is Are Aromatic
Which of the following is an example of heterocyclic aromatic compound. So aromatic compounds are those which meet the following criteria.
Aromatic Compounds And Electrophilic Aromatic Substitution Concise Medical Knowledge
According to Huckel rule for a ring to be aromatic it should have the following properties.

. Correct options are B and D OXAZOLE. 135 ppm 3H triplet 410 ppm 2H. The aromatic heterocycle pyridine is similar to benzene and is often used as a weak base for scavenging protons.
Choose the correct answer. Want to see the full answer. The compound must have a conjugated system with a p orbital at every vertex D.
Which of the following represents an aromatic compound. Which of the following compounds isare aromatic compounds. Aromatic hydrocarbons are hydrocarbons containing sigma bonds and pi electrons between the carbon atom in a ring.
The following compounds undergo electrophilic aromatic substitution EXCEPT arrow_forward What is the structure of the compound with the formula C4H7ClO it has a strong IR signal near 1730 cm-1 the 1H-NMR spectrum is. Thus are aromatic in nature. If has 6 delocalised electrons.
A compound may be aromatic if it obeys the following rules The molecule must be co-planar Complete delocalization of π electron in the ring. Benzene is the simplest aromatic compound. Asked 1 day ago in Chemistry by Sowaiba 712k points class-11.
The compound must be cyclic and planar B. C 6 H 12. Benzene is the archetypical aromatic compound.
Oxazoles are weak bases. Which one of the following statements is not true for a compound to be considered as aromatic. For example 402 gives a two-pi-electron aromatic compound.
The following are the rules for aromaticity. Check out a sample QA here. Introduction to electrophilic aromatic substitution EASSection 162 The G.
Any hydrocarbon can be classified as an aromatic compound provided they follow the Huckel rule. According to the Huckel rule ring having planarity complete delocalization of the pi electrons conjugation of double bonds and the presence of 4 n 2 π electrons in the ring where n is an integer are aromatic compounds. Aromatic compounds are categorized further into two categories ie.
Quick intro to benzeneSection 152 The Structure of Benzene. These are azoles with oxygen and nitrogen separated by one carbon. Among the following compound that is not aromatic.
Which one of the following compounds is aromatic. Aromatic compounds are broadly divided into two categories. Which of the following compounds isare aromatic compounds.
Which one of the following compounds is aromatic. The compound must satisfy Hückels rule -must have 4n 2 electrons. Asked 1 day ago in Chemistry by Sowaiba 712k points class-11.
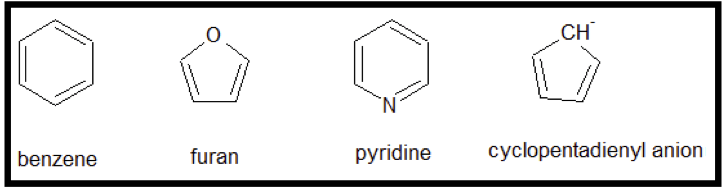
The compounds pyridine 1H-pyrrole and thiophene are heterocyclic aromatic compounds. While benzene and compounds that contain the benzene ring in its chemical structure are known to be aromatic compounds there are other compounds that look slightly different but are aromatic. Describe the structural characteristics.
Presence of 4n2 π electrons in the ring where n is an integer n012This is known as Huckels rule. These are compounds that are not aromatic. The compound must be monocyclic.
So the correct answer is Option D. A The compound must be cyclic. C 6 H 5 OH.
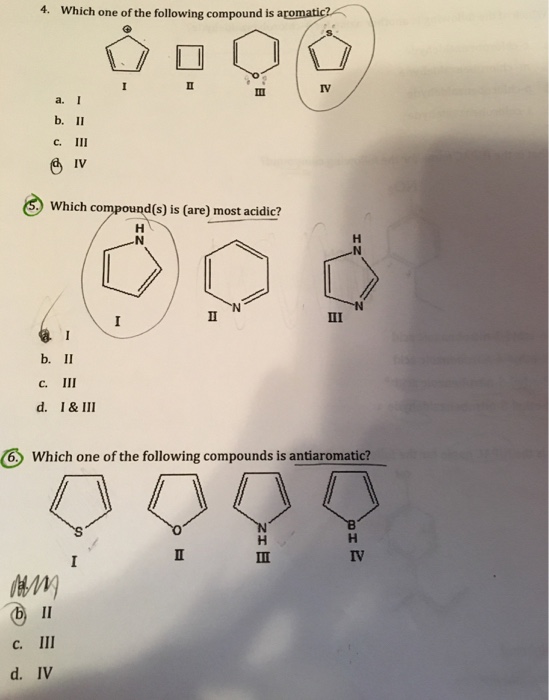
C 6 H 9 OH. Benzenoids are the compounds having at least one benzene ring. A I B II C III D IV.
Oxazoles are heterocyclic aromatic organic compounds. Chapter 16 Lecture Video Part 1Section 161 Electrophilic Aromatic Substitution. Asked 1 day ago in Chemistry by Sowaiba 712k points class-11.
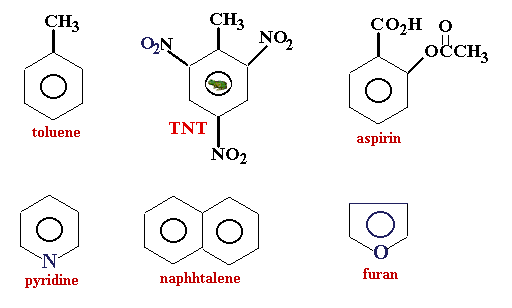
Benzenoids one containing benzene ring and non-benzenoids those not containing a benzene ring for example furan. Want to see the full answer. You could not lonesome going following ebook hoard or library or borrowing from your contacts to edit.
Furan has a planar ring structure. III IV II O IV none of these Expert Solution. Heterocyclic compounds are cyclic compounds with the ring containing carbon and other element the component being oxygen nitrogen and sulfur.
This is because all aromatic compounds must follow Huckels Rule which is 4n2. The structure must be cyclic containing some number of conjugated pi bonds. Aromatic compounds are cyclic conjugated molecules that possess 4n2pi electrons and adopt planar confirmations to allow maximum overlap of the conjugated pi orbitals.
Aromatic compound- The term aromaticity is used to describe a cyclic planar molecule with a ring of resonance bonds that exhibits more stability than other geometric or connective arrangements with the same set of atoms. Note that n in Huckels Rule just refers to any whole number and 4n2 should result in the number of pi electrons an aromatic compound should have. In the last we can conclude that phenol naphthalene and pyridine are aromatic compounds.
Oxazoles are aromatic compounds but less than the thiazoles. Toluene is made of a phenyl group attached to a methyl. Asked 1 day ago in Chemistry by Sowaiba 712k points class-11.
Which one is a heterocyclic compound. Toluene has a similar structure to benzene in addition to a methyl group. Chapter 15 Lecture Video Part 1Section 151 Background.
The aromatic compounds have included the cyclic compounds containing conjugated double bonds with unusually large resonance energies. Up to 10 cash back The correct answer is 8 Annulene. Download Free Aromatic Naming Compounds Practice Problems With Answers Aromatic Naming Compounds Practice Problems With Answers Getting the books aromatic naming compounds practice problems with answers now is not type of challenging means.
Aromatic compounds burn with soofy flame because. Check out a sample QA here. Solve any question of Hydrocarbons with-.
It is planar bond angles120º all carbon atoms in the ring are sp 2 hybridized and the pi-orbitals are occupied by 6 electrons. They are planar and conjugated as both nitrogen and oxygen have a lone pair of electrons which help in the delocalization of. C 6 H 8.
B Every atom in the ring must be conjugated.

Among The Above The Number Of Aromatic Compound S Is Are

What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com

Aromatic Compounds And Their Reactions Organic Chemistry Cheat Sheet Studypk Organic Chemistry Cheat Sheet Organic Chemistry Study Organic Chemistry

Happy Moleculemonday Benzimidazole Is A Heterocyclic Aromatic Organic Compound This Bicyclic Compound Consists Of The Fusi Chemistry Aromatic Molecules

A Reaction Map Pdf For Benzene And Aromatic Compounds Organic Chemistry Books Organic Chemistry Reactions Organic Chemistry

Classification Of Aromatic Antiaromatic And Nonaromatic Compounds Chemistry Lessons Organic Chemistry Study Chemistry
4 12 Heterocyclic Aromatic Compounds Chemistry Libretexts

Organic Chemistry Books Organic Chemistry Chemistry Class

Naming Aromatic Compounds Chemistry Steps

Aromatic Antiaromatic Or Nonaromatic Compounds Chemistry Steps

What Is An Aromatic Compound Definition Example Video Lesson Transcript Study Com

Nitrogen Heterocycles Are Carbon Organic Rings Containing Nitrogen Nitrogen Heterocycles Can Be Formed From Aliphatic Nitro Nitrogen Amino Acids Organic Rings

Aromatic Compounds Study Guide Cheat Sheet Organic Chemistry Study Chemistry Help Teaching Chemistry
Solved 4 Which One Of The Following Compound Is Aromatic Chegg Com

Antiaromaticity And Antiaromatic Compounds Master Organic Chemistry Chemistry Organic Chemistry Chemistry Notes



Comments
Post a Comment